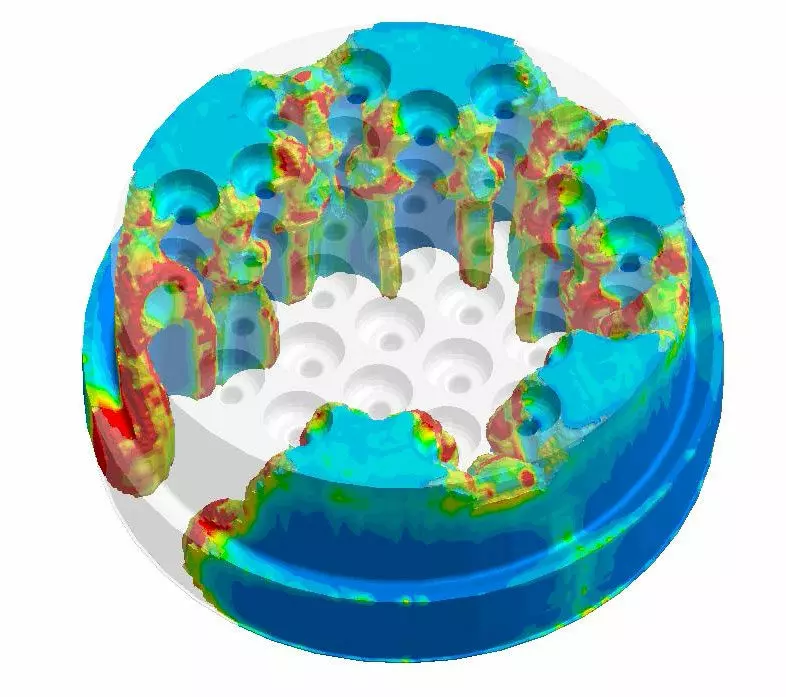
Precision Technologies that Perform
Q creates medical, automotive, industrial, and consumer products that keep people healthy, safe, and productive. Specializing in silicone and other elastomerics, Q leverages world-class, integrated capabilities across the globe to produce mission-critical products designed to perform wherever they’re applied.